
The TCT will be held in Miami Beach, October 22-26, 2012
The Transcatheter Cardiovascular Therapeutics conference (TCT) is the largest U.S. meeting devoted to interventional cardiology (angioplasty, stents, and related procedures) and it starts next week. Organizers are predicting a new attendance record of over 12,000 cardiologists and associated healthcare professionals, as well as members of the device, imaging and pharmaceutical industries, venture capitalists, and press. Speaking of which, yes…I will be there and Angioplasty.Org will be reporting on late-breaking trials, new directions and innovative devices.
The annual meeting is truly international: attendees will be traveling from 70 countries; in fact, this year more than two-thirds of the registrants hail from outside the United States. Continue reading

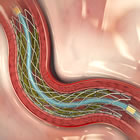
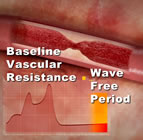

 Earlier today, the opening day of EuroPCR, Dr. Bernard De Bruyne presented
Earlier today, the opening day of EuroPCR, Dr. Bernard De Bruyne presented 
 I’m looking at this morning’s news and I’m seeing headlines like these: “Stents Overused in Stable Heart Patients” (WebMD), “Pills as good as stents for stable heart patients” (Reuters), “No Extra Benefits Are Seen in Stents for Coronary Artery Disease” (New York Times), and “Stents no better than pills for some heart patients” (MassDevice) — and I think I have time-traveled half a decade back to March 2007 when the results of the COURAGE study were presented at the American College of Cardiology Annual Meeting.
I’m looking at this morning’s news and I’m seeing headlines like these: “Stents Overused in Stable Heart Patients” (WebMD), “Pills as good as stents for stable heart patients” (Reuters), “No Extra Benefits Are Seen in Stents for Coronary Artery Disease” (New York Times), and “Stents no better than pills for some heart patients” (MassDevice) — and I think I have time-traveled half a decade back to March 2007 when the results of the COURAGE study were presented at the American College of Cardiology Annual Meeting. 

