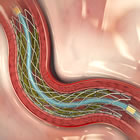
Medtronic’s Resolute Integrity zotarolimus-eluting stent
According to reports yesterday by the Mumbai Bureau of Pharmabiz, and confirmed today by MassDevice, Medtronic has launched its Resolute Integrity Drug-Eluting Stent (DES) in India for the treatment of coronary artery disease (CAD). The Resolute Integrity is the only stent approved by the FDA for use in diabetics, based on a large cohort of diabetic patients in the Resolute clinical program.
Diabetics historically have had higher adverse events when treated with PCI (angioplasty and stents) — but the data from the Resolute trials showed virtually no difference between diabetics and non-diabetics, leading the FDA to approve the indication. Continue reading

 Published “online first” today in the New England Journal of Medicine are two articles, authored by the Democratic and Republican presidential nominees, President Barack Obama and former Massachusetts Governor Mitt Romney, describing their health care platforms and their visions for the future of American health care. The editors of NEJM had asked the nominees for these statements which are brief and concise, summing up the two positions in less than 1,300 words each.
Published “online first” today in the New England Journal of Medicine are two articles, authored by the Democratic and Republican presidential nominees, President Barack Obama and former Massachusetts Governor Mitt Romney, describing their health care platforms and their visions for the future of American health care. The editors of NEJM had asked the nominees for these statements which are brief and concise, summing up the two positions in less than 1,300 words each.
 34 years ago, Andreas Gruentzig performed the first coronary angioplasty. Rather than cutting open the chest, sawing through the sternum and sewing a bypass conduit (harvested from the leg or internal mammary artery) into the coronary artery, he elegantly threaded a balloon catheter to the blockage through a small incision in the femoral (groin) artery, in an awake patient. He then inflated the balloon, compressing the plaque against the arterial wall and opening the artery. The procedure was a total success and his first patient, Adolph Bachmann, is alive and well today! (
34 years ago, Andreas Gruentzig performed the first coronary angioplasty. Rather than cutting open the chest, sawing through the sternum and sewing a bypass conduit (harvested from the leg or internal mammary artery) into the coronary artery, he elegantly threaded a balloon catheter to the blockage through a small incision in the femoral (groin) artery, in an awake patient. He then inflated the balloon, compressing the plaque against the arterial wall and opening the artery. The procedure was a total success and his first patient, Adolph Bachmann, is alive and well today! (

